原料合成实验员
招聘人数:3人
职位描述:
1、 完成药物分子的化学合成设计,实验操作及结构解析;
2、 完成合成工艺线放大与技术转移;
3、根据设计的目标化合物进行文献查阅,合成工艺线路的筛选、设计 ;
4、维护实验室安全和卫生;
5、完成上级交给的其他工作。
任职要求:
1、本科及以上学历,化学、有机化学、药物合成、药物化学等相关专业;
2、熟练操作实验室各种检验、分析仪器;
3、热爱研发工作,踏实肯钻研,具备良好的团队精神。
薪资福利:
1、公司实行5天8小时工作制;
2、员工可享受带薪年休假及国家法定节假日;
3、每年发放年终奖;
4、按国家规定购买城镇社保(五险)及住房公积金;
5、部门不定期团建;
6、设立教育资助金,帮助员工接受继续教育;
7、提供职业发展双通道,管理通道及专业技术通道,给员工提供实现自我的平台。
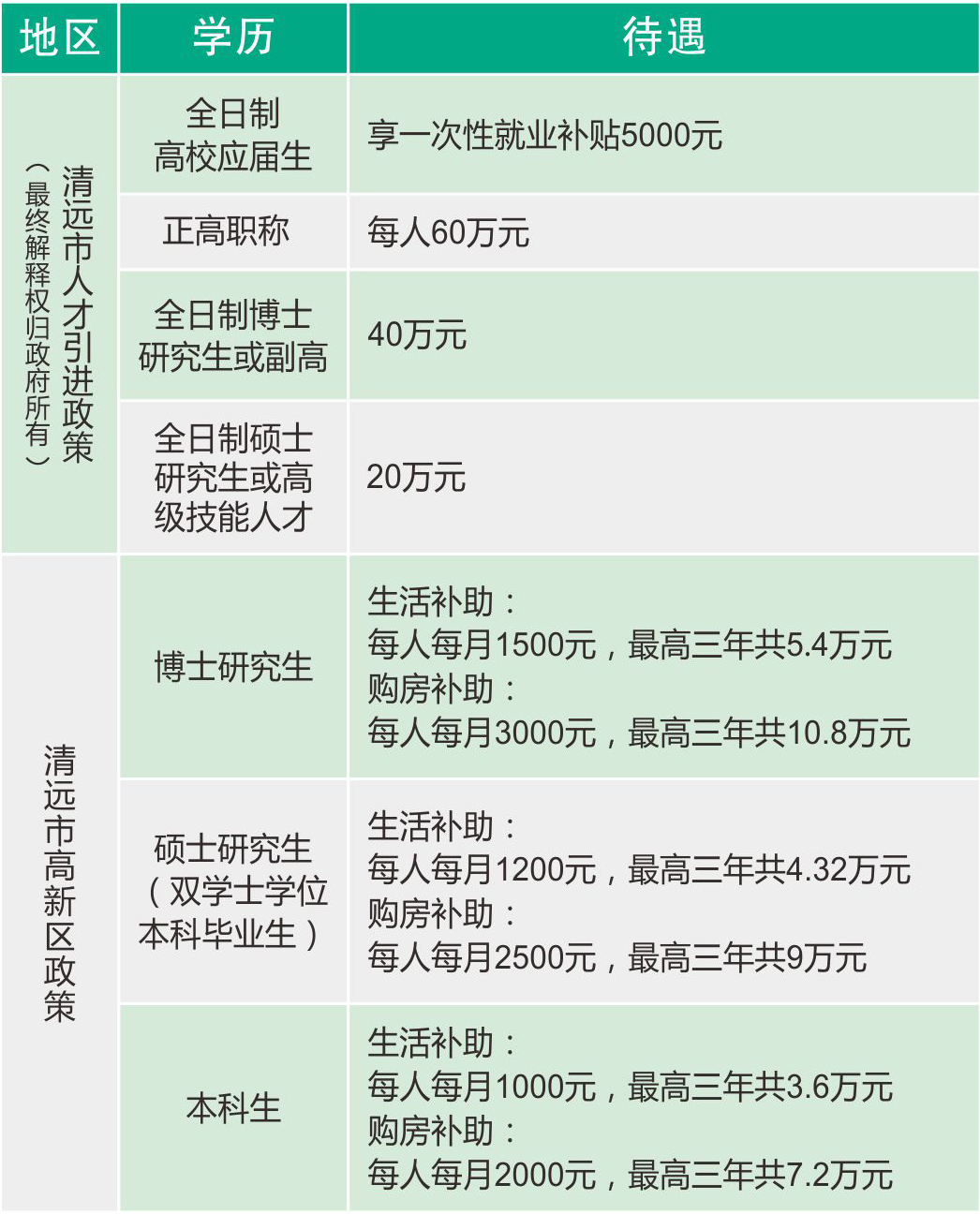
人才政策:
1.清远市人才政策文件导航(关于开展2020年清远市“起航计划”有关人才项目申报工作的通知)
2.高新区人才政策文件导航(关于印发《清远高新区激励优秀青年人才创业就业试行办法》的通知)
![]()
联系我们:
电话:0763-3299666-8106
招聘邮箱:hr@jiabopharm.com
联系人:徐小姐
分析实验员
招聘人数:1人
职位描述:
1、负责研发项目药品(含原料和制剂)的分析检验方法快速开发和优化;
2、负责研发项目药品(含原料和制剂)的分析检验方法的转移;
3、负责研发分析精密仪器的维护和培训;
4、制定和审核质量研究方案等质量文件。
任职要求:
1、本科以上学历,药物分析、食品检验、药物制剂等相关专业;
2、熟练操作液相、气相、质谱或色谱联用仪器,熟悉方法开发和优化流程;
3、至少2年药品分析工作经验。
薪资福利:
1、公司实行5天8小时工作制;
2、员工可享受带薪年休假及国家法定节假日;
3、每年发放年终奖;
4、按国家规定购买城镇社保(五险)及住房公积金;
5、部门不定期团建;
6、设立教育资助金,帮助员工接受继续教育;
7、提供职业发展双通道,管理通道及专业技术通道,给员工提供实现自我的平台。
人才政策:
1.清远市人才政策文件导航(关于开展2020年清远市“起航计划”有关人才项目申报工作的通知)
2.高新区人才政策文件导航(关于印发《清远高新区激励优秀青年人才创业就业试行办法》的通知)
![]()

联系我们:
电话:0763-3299666-8106
招聘邮箱:hr@jiabopharm.com
联系人:徐小姐
新药合成研究员
招聘人数:1人
职位描述:
1、 完成药物分子的化学合成设计,实验操作及结构解析;
2、 完成合成工艺线放大与技术转移;
3、根据设计的目标化合物进行文献查阅,合成工艺线路的筛选、设计 ;
4、维护实验室安全和卫生;
5、完成上级交给的其他工作。
任职要求:
1、本科及以上学历,化学、有机化学、药物合成、药物化学等相关专业;
2、熟练操作实验室各种检验、分析仪器;
3、热爱研发工作,踏实肯钻研,具备良好的团队精神。
薪资福利:
1、公司实行5天8小时工作制;
2、员工可享受带薪年休假及国家法定节假日;
3、每年发放年终奖;
4、按国家规定购买城镇社保(五险)及住房公积金;
5、部门不定期团建;
6、设立教育资助金,帮助员工接受继续教育;
7、提供职业发展双通道,管理通道及专业技术通道,给员工提供实现自我的平台。
人才政策:
1.清远市人才政策文件导航(关于开展2020年清远市“起航计划”有关人才项目申报工作的通知)
2.高新区人才政策文件导航(关于印发《清远高新区激励优秀青年人才创业就业试行办法》的通知)
![]()

联系我们:
电话:0763-3299666-8106
招聘邮箱:hr@jiabopharm.com
联系人:徐小姐

